Answer:
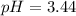
Step-by-step explanation:
Hello.
In this case, since the formic acid (HCHO2) is a weak acid and it is mixed with its conjugate base (CHO2⁻) in the form of NaCHO2, we can compute the pH via the Henderson-Hasselbach equation:
![pH=pKa+log(([Base])/([Acid]) )](https://img.qammunity.org/2021/formulas/chemistry/college/km74gaxjix359lr99r0my3yacfx5dsp8u5.png)
Whereas the concentration of both acid and base are computed considering final total volume of 75.0 mL after adding the volume of each solution as shown below:
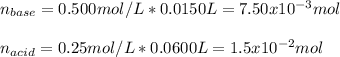
And the resulting concentrations upon the final volume:
![[Base]=(7.50x10^(-3)mol)/(0.0750L)=0.1M](https://img.qammunity.org/2021/formulas/chemistry/college/jtlhhhocnuon9gdo8gs6ic7ay9vik480yh.png)
![[Acid]=(1.5x10^(-2)mol)/(0.0750L)=0.20M](https://img.qammunity.org/2021/formulas/chemistry/college/3kxahblk01bz1rwnvx13gaf47pc7w4xhro.png)
Thus, the pH is:
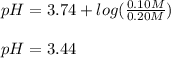
Best regards.