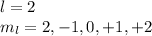Answer:
(1) The wavelength of the proton is 1.366 x 10⁻¹⁵ m
(2) 2p( l = 1, ml = -1,0,+1)
3s( n = 3, l = 0, ml = 0)
5d ( l = 2, ml = -2,-1,0,+1,+2)
Step-by-step explanation:
Given;
mass of the proton; m = 1.673 x 10⁻²⁴ g = 1.673 x 10⁻²⁷ kg
velocity of the proton, v = 2.9 x 10⁸ m/s
The wavelength of the proton is calculated by applying De Broglie's equation;
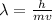
where;
h is Planck's constant = 6.626 x 10⁻³⁴ J/s
Substitute the given values and solve for wavelength of the proton;
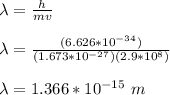
(2) the values for the quantum numbers associated with the following orbitals is given by;
n, which represents Principal Quantum number
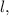 which represents Azimuthal Quantum number
which represents Azimuthal Quantum number
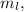 which represents Magnetic Quantum number
which represents Magnetic Quantum number
(a) 2p (number of orbital = 3):
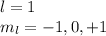
(b) 3s (number of orbital = 1):
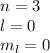
(c) 5d (number of orbital = 5)