Answer:
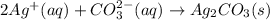
Step-by-step explanation:
Hello.
In this case, since the reaction is not given but a typical precipitation of silver from silver nitrate could be represented by its reaction with a soluble metallic sulfide or carbonate since silver carbonate and silver sulfide are insoluble, we can use for instance sodium carbonate to obtain:
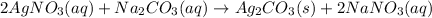
Thus, since, silver nitrate, sodium carbonate and sodium nitrate are fully ionized by silver carbonate remains solid, the complete ionic equation is:
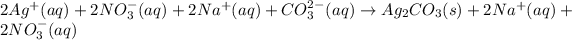
Then, since nitrate and sodium ions are the spectator ions since they are at both reactants and products, the net ionic equation turns out:
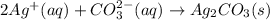
Best regards!