Answer:
Chromium (III) bromide.
Step-by-step explanation:
Hello.
In this case, for the reaction:
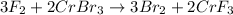
Since there is a 3:2 mole ratio between fluorine (molar mass = 38.0 g/mol) and chromium (III) bromide (molar mass = 291.7 g/mol) we can compute the available moles of fluorine and the moles of fluorine consumed by 22.22 g chromium (III) bromide in order to realize whether it is the limiting reactant or not:
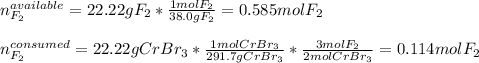
In such a way, since just 0.114 off 0.585 moles of fluorine are consumed, we conclude it is in excess and the chromium (III) bromide is the limiting reactant, it means it gets completely used up.
Best regards!