Answer: The answer is C. 0.1MCaCl2
Step-by-step explanation:
We have AgCl whose least solubility is to be determined at 25degree Celsius
so upon looking at the options given we observe C 0.1MCaCl2 and D 0.1MHCl both have chlorine ions present and we know that here common ion effect will happen that causes decreases the solubility of the AgCl here
but when Calcium chloride dissociates it gives 2 moles of chlorine ions
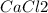 →
→
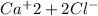
so chlorine ion will be present in more amount
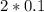 =
=
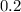 as compared to the chlorine produced by HCl
as compared to the chlorine produced by HCl
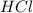 →
→
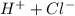 only 1 mole of chlorine is produced.
only 1 mole of chlorine is produced.
Hence AgCl will be least soluble in 0.1M CaCl2