Answer:
The concentration (in mM units) of vitamin C in the juice is 0.64mM.
Step-by-step explanation:
Let us calculate -:
Concentration of sample =>
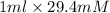
= 29.4 mmol => 29.4mmol/50ml
= 0.588mM
plug in formula =>
![([final concentration])/([v_1/v_2]) *[final concentration]+[concentration of sample])](https://img.qammunity.org/2021/formulas/chemistry/college/lb5go95tb6gdsy9u6kwctfv7ajo5vkx2tj.png) or
or
![([x])/([v_1/v-2]) *[ x]+[concentration of sample])](https://img.qammunity.org/2021/formulas/chemistry/college/wazbbicsrdl9poxnfklug7sjhm2um4cty3.png)
= [2.02 micro-Amber/3.79micro-Amber]
plug in and simplify
1.81x = 1.17
x = 0.64mM
Hence , the answer is 0.64mM.