Final answer:
The compound formed from potassium and acetate is potassium acetate, with the formula
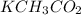 . The compound formed from potassium and chromate is potassium chromate, with the formula
. The compound formed from potassium and chromate is potassium chromate, with the formula
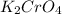 .
.
Step-by-step explanation:
The compound that forms from potassium and acetate is potassium acetate, which has the chemical formula
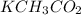 . Potassium is a cation with a charge of +1, and acetate is an anion with a charge of -1. The formula is determined by balancing the charges of the cation and anion.
. Potassium is a cation with a charge of +1, and acetate is an anion with a charge of -1. The formula is determined by balancing the charges of the cation and anion.
The compound that forms from potassium and chromate is potassium chromate, which has the chemical formula
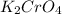 . Potassium is a cation with a charge of +1, and chromate is an anion with a charge of -2. Again, the formula is determined by balancing the charges.
. Potassium is a cation with a charge of +1, and chromate is an anion with a charge of -2. Again, the formula is determined by balancing the charges.