Answer:
20.3%
Step-by-step explanation:
Given mass of impure ore = 896g and mass of pure iron to be 182g after purification,
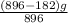 × 100
× 100
= 79.7%
in subtracting the weight of impure mass from that of the pure sample, we are left with the mass of the impurities alone.
This means the mass percent of pure iron in this iron ore sample is 20.3%.
Let me know if this was helpful.