Answer:
2.88 g
Step-by-step explanation:
First, let us look at the balanced equation of the reaction:

From the equation, 1 mole of CaO reacts with 1 mole of H2O to produce 1 mole of
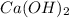 .
.
mole = mass/molar mass
4.50 g of CaO = 4.50/56.08 = 0.08 mole
4.34 g of H2O = 4.34/18.02 = 0.24 mole
The ratio of moles of CaO and H2O should be 1:1, hence, H2O is in excess.
Excess mole of H2O = 0.24 - 0.08 = 0.16 mole
mass of 0.16 mole of H2O = mole x molar mass = 0.16 x 18.02 = 2.88 g
Therefore, 2.88 g of the excess reagent (water) would remain after the reaction is complete.