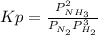Answer:
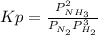
Step-by-step explanation:
For the reaction at equilibrium:
N₂ (g) + 3 H₂(g) ⇄ 2 NH₃ (g)
Since this is an equilibrium in the gas phase, the equilibrium expression is obtained from the partial pressures (P) of the products (NH₃) and reactants (N₂ and H₂) raisen to their stoichiometric coefficients: