Answer:
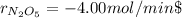
Step-by-step explanation:
Hello.
In this case, since the chemical reaction is:
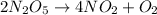
We can write the rate ratio between N2O5 and NO2 as shown below:
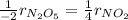
Since N2O5 is consumed and NO2 produced at a rate of 8.00 mol/s, therefore, the rate of disappearance is N2O5 is:
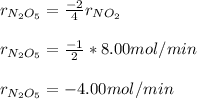
Which is negative since N2O5 is a reactant.
Best regards!