Answer:
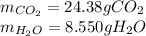
Step-by-step explanation:
Hello.
In this case, since the combustion of C7H12 which is probably heptyne (molar mass = 96 g/mol) is:
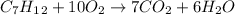
In which the products are carbon dioxide (molar mass = 17 g/mol) and water (18 g/mol) that are in a 7:1 and 6:1 mole ratio with the heptyne, respectively; thus, the yielded mass of both carbon dioxide and water is:
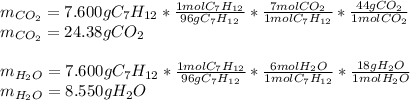
Best regards.