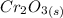Answer:
The balanced chemical equation for the reaction can be computed as:
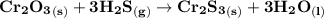
2.103 moles of
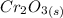 is being required.
is being required.
Step-by-step explanation:
The balanced chemical equation for the reaction can be computed as:
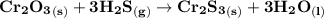
Recall that numbers of moles =
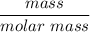
∴
number of moles of Cr2S3 =
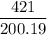
number of moles of Cr2S3 = 2.103 moles
It obvious from the balanced equation that:
1 mole of
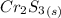 requires 1 mole of
requires 1 mole of
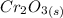
Thus; 2.103 moles of
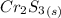 = 2.103 × (1/1) moles of
= 2.103 × (1/1) moles of
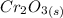
= 2.103 moles of