Answer:
![Kc=([SF_6]^8)/([F_2]^2^4)](https://img.qammunity.org/2021/formulas/chemistry/college/tjikfatlvwaitpcq4v7j3v2dmb6o0266hg.png)
Step-by-step explanation:
Hello.
In this case, for the undergoing chemical reaction:
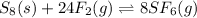
We consider the law of mass action in order to write the equilibrium expression yet we do not include S8 as it is solid and make sure we power each gaseous species to its corresponding stoichiometric coeffient (24 for F2 and 8 for SF6), thus we obtain:
![Kc=([SF_6]^8)/([F_2]^2^4)](https://img.qammunity.org/2021/formulas/chemistry/college/tjikfatlvwaitpcq4v7j3v2dmb6o0266hg.png)
Best regards!