Answer:
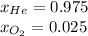
Step-by-step explanation:
Hello.
In this case, since we know the mass of both helium and oxygen, in order to obtain the mole fractions we first need the compute the moles by using their atomic masses, 4.00 g/mol and 32.00 g/mol respectively as shown below:
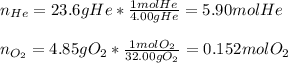
Therefore, the mole fractions are:
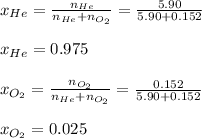
Best regards!