Answer:
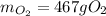
Step-by-step explanation:
Hello.
In this case, for the reaction:
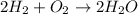
The correct way to compute the oxygen that reacted is by considering the mass of hydrogen, 58.9 g, its molar mass, 2.02 g/mol, the 2:1 mole ratio between hydrogen and oxygen and the atomic mass of that gaseous oxygen 32.00 g/mol; therefore we use the following stoichiometric procedure:
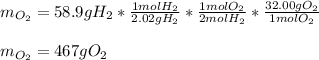
The same result could have been obtained by using the mass of water since the law of conservation of mass is obeyed here.
Best regards!