D. Plutonium-238 is the most stable isotope
Further explanation
Radioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles (such as alpha (α), beta (β), gamma (γ) particles)
General formulas used in decay:
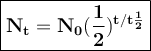
T = duration of decay
t 1/2 = half-life
N₀ = the number of initial radioactive atoms
Nt = the number of radioactive atoms left after decaying during T time
Half-life shows the time it takes for an atom to get half of its initial mass (to decay to half of its initial mass)
The longer the half-life indicates the stability of the atom
Half-life (hr) of isotopes :
Polonium-210 : 3,310
Uranium-237 : 162
Francium-227 : 0.04
Plutonium-238 : 768,000
The half-life value above shows that Plutonium-238 has the longest time, making it the most stable