Answer:
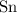 ,
,
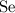 ,
,
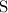 ,
,
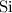 .
.
Step-by-step explanation:
The relative atomic mass of an element is numerically equal to the mass (in grams) of one mole of its atoms. This quantity can help estimate the number of moles of atoms in each of these four
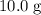 samples.
samples.
Look up the relative atomic mass for each of these four elements (on a modern periodic table.)
The relative atomic mass of
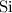 is (approximately)
is (approximately)
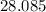 . Therefore, the each mole of silicon atoms would have a mass of approximately
. Therefore, the each mole of silicon atoms would have a mass of approximately
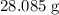 . How many moles of silicon atoms would there be in a
. How many moles of silicon atoms would there be in a
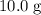 sample?
sample?
Given:
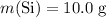 .
.
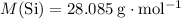 .
.
Number of mole of silicon atoms in the sample:
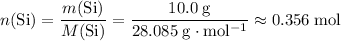 .
.
Similarly:
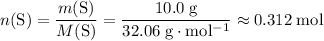 .
.
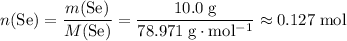 .
.
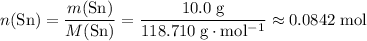 .
.
Therefore, among these
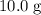 samples:
samples:
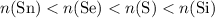 .
.
It is not a coincidence that among these four samples, the one with the fewest number of atoms corresponds to the element with the largest relative atomic mass.
Consider two elements, with molar mass
 and
and
 each. Assume that
each. Assume that
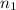 moles and
moles and
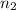 moles of atoms of each element were selected, such that the mass of both samples is
moles of atoms of each element were selected, such that the mass of both samples is
 . That is:
. That is:
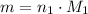 .
.
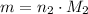 .
.
Equate the right-hand side of these two equations:
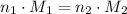 .
.
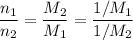 .
.
In other words, the number of moles atoms in two equal-mass samples of two elements is inversely proportional to the molar mass of the two elements (and hence inversely proportional to the formula mass of the two elements.) That explains why in this question, the sample containing the smallest number of atoms corresponds to element with the largest relative atomic mass among those four elements.