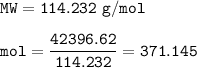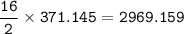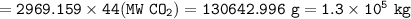Mass of CO₂ = 1.3 x 10⁵ kg
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
2C₈H₁₈ + 25O₂⇒ 16CO₂ + 18H₂O
16 gallon = 60566,6 ml