Answer:

Step-by-step explanation:
The density of an object can be found by dividing the mass by the volume.
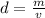
The mass of the cylinder is 14.98 grams.
The volume of the cylinder is 2.10 milliliters.
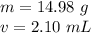
Substitute the values into the formula.
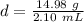
Divide.
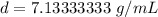
Round to the nearest hundredth. The 3 in the thousandth place tells us to leave the 3 in the hundredth place.
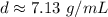
The density of the cylinder is about 7.13 grams per milliliter.