Answer:
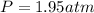
Step-by-step explanation:
Hello!
In this case, since we are asked to compute the pressure of 0.175 moles of the given gas at 22.0 °C occupying a volume of 2175 mL, we can use the ideal gas equation as shown below:

Whereas our unknown is P and we solve for it as shown below:
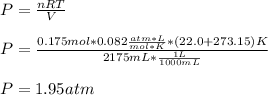
Take into account that based on the given R units, the temperature must be in Kelvins and the volume in liters.
Best regards!