Answer:
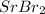
Step-by-step explanation:
Chemical formulas are a way to show the elements and the amount of each element present in a compound.
Element Symbol
To write a chemical formula you need to use their chemical symbols. So, we need to find the symbols for strontium and bromide.
- Strontium is Sr
- Bromine is Br
Charges
However, elements do not combine in random ratios. The ratios are based on charges so that all compounds have a neutral charge. Elements will have different subscripts to ensure that compounds have a neutral overall charge. So, to correctly write the formula we need to know the charges Sr and Br have when they became ions.
- Sr gains a charge of +2
- Br gains a charge of -1
To cancel the positive 2 out, we must have 2 bromines. That means the final formula for strontium bromide is
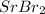 . Now, the compound has a charge of 0.
. Now, the compound has a charge of 0.