Answer:
0.22 moles
Step-by-step explanation:
Moles are a measurement used in chemistry that describes the amount of an atom based on the number of atoms. One mole is equal to
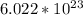 , which is the number of atoms in 12 grams in Carbon-12.
, which is the number of atoms in 12 grams in Carbon-12.
Mass of Copper
Before finding the moles of copper, we need to know the mass. We are told that a door handle is 140g, but only 10% is copper.
To find 10% for 140 we can multiply 140 * 0.1.
This means there are 14 grams of copper.
Moles of Copper
Now, we can use dimensional analysis to convert grams to moles. To do this we need to know how many grams of copper are in 1 mole. This number is called the molecular mass and can be found on the periodic table. This number is usually directly below the element's symbol and is the only number with decimal points (not all periodic tables will have the exact same molecular mass because the number can change, but they will be close).
The molecular mass for copper is 63.55. Next, we can set up a dimensional analysis conversion. Dimensional analysis is when you multiply some value by a ratio of 2 different values with 2 different units. This may sound confusing but it makes more sense when you do it.
When setting up an expression like this, make sure that the units cancel out so you are only left with the one you want. Units will cancel out if one is in the numerator of a ratio and the other is in the denominator.
Next, multiply this out (which is equivalent to dividing 14/63.55)
So, when rounded to significant figures, there are 0.22 moles in 14 grams of copper.