Answer:
Ans : 22.86 gm of Aluminium
Solution,
3FeO + 2Al ---> 3 Fe +
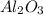
Given 3 2
Find 1.27 x
By the given gms of FeO find its mole and put the moles in the find,
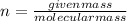
Given mass of FeO is 91.8
Molecular mass of FeO is 71.8
n =
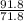
n = 1.27 moles of FeO
Now solve for x,
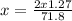
x = 0.85 moles
These are just moles of Aluminium that we need to react with FeO, so to find Grams of Aluminium we need,
Mass = moles x molecular mass
Mass = 0.85 x 27(atomic mass of Al)
Mass = 22.86 grams of aluminium is what we need