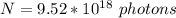Answer:
The value is
Step-by-step explanation:
From the question we are told that
The power rating of the bulb is P = 25 W
The efficiency is
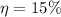
The wavelength is
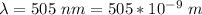
Generally the energy of the photon emitted is mathematically represented as
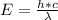
Here h is the planks constant with a value
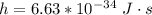
c is the speed of light with value
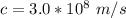
So
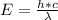
=>
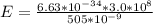
=>
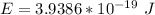
Generally we are told that 1 W = 1 J/s
Hence 25 W = 25 J
Generally the amount of this energy converted to light is
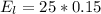
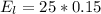
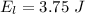
Generally the number of photons that are emitted by the light bulb per second is mathematically represented as
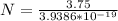
=>