Answer:
4.5 moles
Step-by-step explanation:
The given reaction is

Moles of calcium nitrate is 1.5
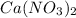 produces 2 moles of
produces 2 moles of
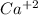 and 1 mole of
and 1 mole of
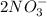
So the total moles of the ions of the products are 3
Therefore in the solution the moles of ions present are
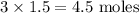
Moles of ions present in solution is 4.5.