Answer:
26.08 g
Step-by-step explanation:
The equation of the reaction for the decomposition of mercury(II) oxide is:
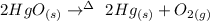
Number of moles of HgO = mass/molar mass
Number of moles of HgO = 7.06 × 10² g/ 216.59 g/mol
Number of moles of HgO = 3.26 moles
From the above equation, two moles of mercury (II) oxide decompose to produce two moles of mercury and one mole of oxygen.
Thus, the number of moles of O₂ produced = 1/2 × 3.26 moles
the number of moles of O₂ produced = 1.63 moles
The mass of oxygen O₂ produced = 1.63 moles × 16.0 g/moles
The mass of oxygen O₂ produced = 26.08 g