Answer:
The heat gained by water is 41,916 kJ
Step-by-step explanation:
Given;
mass of cast Iron,
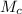 = 200 kg
= 200 kg
mass of water,
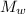 = 400 kg
= 400 kg
initial temperature of the cast iron,
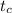 = 500 ⁰C
= 500 ⁰C
initial temperature of the water,
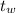 = 20 ⁰C
= 20 ⁰C
At thermal equilibrium, the heat lost by the cast iron, is equal to heat gained by the water

The heat gained by water is given by;
Qw = 400 x 4200(44.95 - 20)
Qw = 41916000 J
Qw = 41,916 kJ
Therefore, the heat gained by water is 41,916 kJ