Answer:
- The limiting reagent is Sn(NO₂)₄
Step-by-step explanation:
The reaction that takes place is
- 3Sn(NO₂)₄ + Pt₃N₄ → Sn₃N₄ + 3Pt(NO₂)₄
Now we write down the molar mass of Sn(NO₂)₄, Pt₃N₄ and Sn₃N₄:
In order to determine the limiting reagent, we calculate the moles of Sn(NO₂)₄ and Pt₃N₄:
- 102 g Sn(NO₂)₄ ÷ 302.71 g/mol = 0.337 mol Sn(NO₂)₄
- 80.2 g Pt₃N₄ ÷ 251.08 g/mol = 0.319 mol Pt₃N₄
From the balanced reaction we know that 1 mole of Pt₃N₄ would react completely with 3 moles of Sn(NO₂)₄, so 0.319 moles of Pt₃N₄ would require (0.319*3) 0.957 moles of Sn(NO₂)₄ to completely react.
There are not so many Sn(NO₂)₄ moles so Sn(NO₂)₄ is the limiting reagent.
To calculate the yield, first we calculate the moles of Sn₃N₄ produced:
- 40.2 g Sn₃N₄ ÷ 412.13 g/mol = 0.098 mol Sn₃N₄
And we compare it with the amount that the limiting reagent (Sn(NO₂)₄) could produce:
- 0.337 mol Sn(NO₂)₄ *
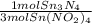 = 0.112 mol Sn₃N₄
= 0.112 mol Sn₃N₄
So the percent theoretical yield is:
- 0.098 / 0.112 * 100% = 87.5%