Answer:
- 775.5 kJ/mol
Step-by-step explanation:
Given that:
The enthalpy of formation of MX is
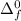 = - 421 kJ/mol
= - 421 kJ/mol
The enthalpy of sublimation of MM is
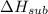 = 155 kJ/mol
= 155 kJ/mol
The ionization energy of MM is = 401 kJ/mol
The electron affinity of XX is
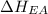 = -321 kJ/mol
= -321 kJ/mol
The bond energy of X2 is BE = 239 J/mol
The process for each above component is shown below:
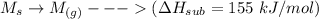
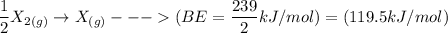
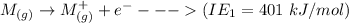
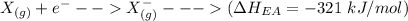
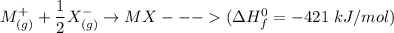
Thus, the overall reaction is:
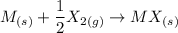
The overall energy is:
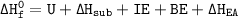
- 421 kJ/mol = U + 155 kJ/mol + 401 kJ/mol + 119.5 kJ/mol + (-321 kJ/mol)
- 421 kJ/mol = U + 354.5 kJ/mol
- U = 421 kJ/mol + 354.5 kJ/mol
- U = 421 kJ/mol + 354.5 kJ/mol
- U = 775.5 kJ/mol
U = - 775.5 kJ/mol
Thus, the lattice energy of MX (U) = - 775.5 kJ/mol