Answer:
- Molarity of the solution is - 0.138 M glucose or moles/litre.
- Number of moles of glucose contained in 237ml of glucose is - 0.0327 moles glucose.
- Volume of the glucose containing 0.79 moles of glucose is - 0.572 L of glucose .
Step-by-step explanation:
- To find the molarity of the solution -:
we know , molar mass of glucose is 18.08 g
now , moles of glucose =
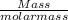
=
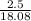
= 0.138 moles of glucose .
Molarity =
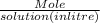
=
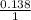
=0.138 M glucose or moles/litre.
- To find number of moles of glucose contained in 237 ml glucose solution-:
Number of moles of glucose contained in 237ml of glucose =
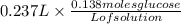
= 0.0327 moles glucose.
- To find the volume of the glucose containing 0.79 moles of glucose -:
=
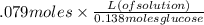 (by using the molarity formula)
(by using the molarity formula)
= 0.572 L of glucose .
Hence ,
- Molarity of the solution is - 0.138 M glucose or moles/litre.
- Number of moles of glucose contained in 237ml of glucose is - 0.0327 moles glucose.
- Volume of the glucose containing 0.79 moles of glucose is - 0.572 L of glucose .