Given :
A reaction , HNO3(aq) + KOH(aq) -> KNO3(aq) + H2O(l)
To Find :
Volume of 0.50 M HNO3 is required to completely react with 16.0 mL of 1.250 M KOH .
Solution :
From the equation we can see that 1 mol of HNO3 react with 1 mol of KOH.
So,
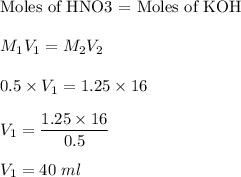
Therefore, volume of HNO3 required is 40 ml.
Hence, this is the required solution.