Answer:
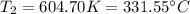
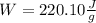
Step-by-step explanation:
Hello!
In this case, for this compression of air from 101 kPa to 1200 kPa, we should first realize that equation relating P and T is:
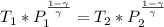
Whereas:
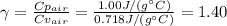
That is why the final temperature is:
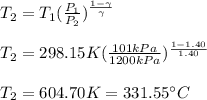
Moreover, for the work done, since the process is adiabatic, no heat is in the equation:
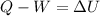
But as the work is done on the system we can write:
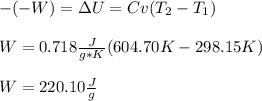
Best regards!