Answer:
a)4.15 years
b)22.18 years
c)188.53 years
d)3027.66 years
Explanation:
Formula :
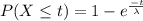
So,
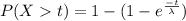
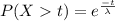
We are given that The half-life of such a persistent poison is that time beyond which the probability is .50 that a particular molecule will remain toxic i.e. P(X>t)=0.5
So,
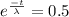
Taking natural log both sides
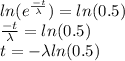
a)
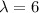
t=-(6) ln(0.5)=4.15 years
b)
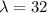
t=-(32) ln(0.5)=22.18 years
c)
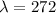
t=-(272) ln(0.5)=188.53 years
d)
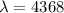
t=-(4368) ln(0.5)=3027.66 years