Answer:
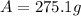
Step-by-step explanation:
Hello.
In this case, since the radioactive decay is computed via:
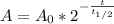
For the initial amount of Ra-226 (300 g), once 200 years have passed, the remaining mass is:
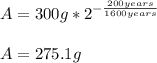
This is, considering that the half-life is 1600 years, it means that the mass of Ra-226 is decreased.
Best regards.