Answer:
0.4235 g of glycine amide should be added
Step-by-step explanation:
From the given information:
The equation of the reaction can be illustrated as:
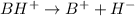
where:
mass of glycine amide hydrochloride = 1.00 g
Suppose x be the amount of (in grams) of glycine amide that is required to be added; Then:
![[BH^+] = (1.00 \ g)/(110.54 \ gmol * 0.100 L)](https://img.qammunity.org/2021/formulas/chemistry/college/2groxztk6iw1dwwaxgiwdm5tuh7m463a4e.png)
![[BH^+]=](https://img.qammunity.org/2021/formulas/chemistry/college/w39yqlwstqfw9uic2he7fp1tvo1d6z4oix.png) 0.0905 M
0.0905 M
So, For [B} i.e, for glycine amide
![[B] = (x \ g)/(74.083 \ gmol * 0.100 L)](https://img.qammunity.org/2021/formulas/chemistry/college/zkqyg7qhqfo04161jjqdfpq91ifg66lp5j.png)
[B] = 0.135x M
∴
![([B])/([BH^+])=(0.135x \ M)/(0.0905 \ M )}](https://img.qammunity.org/2021/formulas/chemistry/college/lx3c1l1kkqjy2oqzdrt737qzgbbeyw88nq.png)
![([B])/([BH^+])=1.49x](https://img.qammunity.org/2021/formulas/chemistry/college/g0f39gdtkepuoc9relh6x42mksa8jdudt6.png)
The log of the above question can be computed as:
![log _(10)([B])/([BH^+])=log _(10)1.49x](https://img.qammunity.org/2021/formulas/chemistry/college/1mk8lhybz2ryt4tmzclaysvg2g3equpj6h.png)
![pH = pK_a + log _(10) ([B])/([BH^+])](https://img.qammunity.org/2021/formulas/chemistry/college/fbcq5govaz8qkhhf07u2qbsef14aml76lj.png)
8.00 = 8.20 +
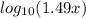
8.00 - 8.20 =
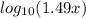
-0.20 =
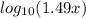
1.49 x =
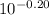
1.49 x = 0.631
x = 0.631/1.49
x = 0.4235 g