Answer:
The new freezing point of water is -0.476°C.
Step-by-step explanation:
Given that,
Mass of water = 4.62 kg
Mass of CaBr₂ = 236.5 g
We need to calculate the molality of solution
Using formula of molality
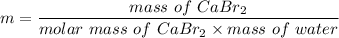
Put the value into the formula
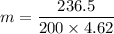
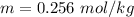
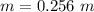
We need to calculate the depression in freezing point of water
Using formula of freezing point
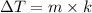
Where, k = depression constant
m = molality of solution
Put the value into the formula
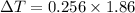
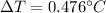
We know that,
The standard freezing point of water is 0°C
We need to find the new freezing point of water
Using formula for freezing point of water
Freezing point of 4.62 kg of water = freezing point of water-depression in freezing point of water
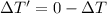
Put the value into the formula
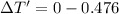
Hence, The new freezing point of water is -0.476°C.