Answer:
7 for pure water.
13.9 when 0.0200 mol of NaOH have been added.
Step-by-step explanation:
Hello.
In this case, since the pH is computed via:
![pH=-log([H^+])](https://img.qammunity.org/2021/formulas/chemistry/college/80k0jnij8v7qd0dtehezpuvp9exa33y466.png)
For pure water it is widely known that its pH is 7 as it is the universal neutral substance. However, once 0.0200 mol of sodium hydroxide has been added, we can notice that the concentration hydrogen ions is changed due to the presence of hydroxide ions, that is why, since NaOH is a base, we are going to compute the pOH rather than the pH at first based on the resulting concentration of NaOH:
![[NaOH]=(0.200mol)/(0.2500L)=0.8M](https://img.qammunity.org/2021/formulas/chemistry/college/5e99lf9afuautrhxmozhkulj2zl1ikr72y.png)
Moreover, since NaOH is a strong base, it is totally dissociated into Na⁺ and OH⁻ ions, that is why the concentration hydroxide ions is 0.8 M as well. In such a way, the pOH is then:
![pOH=-log([OH^-])=-log(0.8000)=0.09691](https://img.qammunity.org/2021/formulas/chemistry/college/c8m3sktayjzzocolgieh0xhohnxhdyn8lp.png)
And the pH:
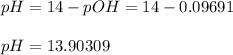
Best regards.