Answer:
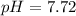
Step-by-step explanation:
Hello,
In this case, considering that TRIS is a weak base and the TRIS hydrochloride its conjugate acid with a pKa of about 8.08, we can use the Henderson-Hasselbach equation in order to compute the pH of the described solution:
![pH=pKa+log(([TRIS])/([TRIS-HCl]) )](https://img.qammunity.org/2021/formulas/chemistry/college/9arf6vnqf1frndr5crqyuu4q5mj83av9e4.png)
Next, we compute the concentrations of TRIS and TRIS hydrochloride as shown below (mol/L):
![[TRIS]=(7.0g/(121.14g/mol))/(0.250L)=0.13M](https://img.qammunity.org/2021/formulas/chemistry/college/lna9t8ct9hi8ad5561wxc52id9ca2o7o9n.png)
![[TRIS-HCl]=(12.0g/(157.60g/mol))/(0.250L)=0.305M](https://img.qammunity.org/2021/formulas/chemistry/college/nbk4hoeg9okwqryyfdk2lrhcetgwsvhtg1.png)
Then, the pH:
Best regards!