We know that pH is a measure of acidity or alkalinity that indicates the amount of hydrogen ions present in a solution or substance which is defined as the negative base 10 logarithm of the activity of hydrogen ions. Which states -
- pH= - log [H⁺]= - log [H₃O⁺]
As per question, we are given a pH value which is 7.91 for a solution and have asked to find out the hydronium ion concentration. Since we have the value of pH, so we can put into the formula and solve for [H₃O⁺] -
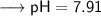
![\:\:\:\:\:\:\longrightarrow \sf - log [H₃O⁺] = 7.91\\](https://img.qammunity.org/2021/formulas/chemistry/college/pni2od93zwstctd1tc4v7hn1pm0ib9nx96.png)
![\:\:\:\:\:\:\longrightarrow \sf [H₃O⁺] = 10^(-7.91)\\](https://img.qammunity.org/2021/formulas/chemistry/college/gun68beio46kvor39f101bsvh67cea77i4.png)
![\:\:\:\:\:\:\longrightarrow \sf \underline{[H₃O⁺] = 1.2302 * 10^(-8)\:M} \\](https://img.qammunity.org/2021/formulas/chemistry/college/fykrdqr08ngkty13ruvcsy4j2z8nsax2z0.png)
Therefore, the hydronium concentration for the solution is
