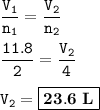Volume (in L) of Carbon dioxide : 23.6 L
Further explanation
Avogadro's hypothesis:
In the same T,P and V, the gas contains the same number of molecules
So the ratio of gas volume will be equal to the ratio of gas moles
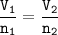
Combustion of ethane :
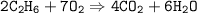
mol ratio of C₂H₆ : CO₂ ⇒ 2 : 4
so the volume of CO₂ :