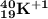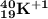
Further explanation
The elements in nature have several types of isotopes
Isotopes are atoms whose no-atom has the same number of protons while still having a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
The element has : 19 protons, 21 neutrons, and 18 electrons.
atomic mass = protons+neutrons = 19+21=40
atomic number=number of protons=19
charge of elements = protons-electrons = 19-18=+1
The element in the periodic system having the atomic number 19 is K
So symbol of element