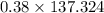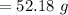Answer:
The answer is "52.18 g"
Step-by-step explanation:
Phosphorus mass = 12.39
molar mass of

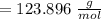
Phosphorus moles =
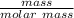
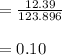
mass = 40.75 g
 molar mass
molar mass
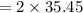
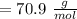
 moles =
moles =
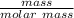
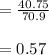
The balanced equation is:
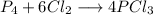
In the step1:
Consider reactor limiting the reaction current is less than necessary quantity as per the balanced equation is considered reactor limiting
The equilibrium equation:
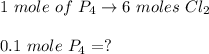
Moles of
 required = 0.6 moles
required = 0.6 moles
But only
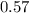 ,
,
 moles are less than the amount needed Thus,
moles are less than the amount needed Thus,
 is reactant restricted
is reactant restricted
In the step2:
Find
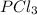 Theoretical performance
Theoretical performance
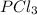 the amount formed when the reactant is fully restricted
the amount formed when the reactant is fully restricted
From equilibrium
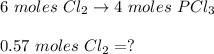
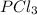 shaped mass =
shaped mass =
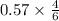
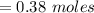
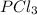 shaped mass =
shaped mass =
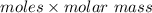
Mass shaped by
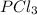 =
=