Answer:
The pH in a solution prepared by continuously bubbling SO₂ is 1.03.
Step-by-step explanation:
Searching in google the reaction is:
SO₂(g) + H₂O(l) ⇄ H₂SO₃(ac) K = 1.33
The constant is:
![K = \frac{[H_(2)SO_(3)]}{P_{SO_(2)}} = 1.33](https://img.qammunity.org/2021/formulas/chemistry/college/38emzoyg6z0klwc8t9xkdt9q1iho4ajxzj.png)
Having that K = 1.33 and P(SO₂) = 0.500 atm, the concentration of H₂SO₃ is:
![[H_(2)SO_(3)] = K*P_{SO_(2)} = 1.33*0.500= 0.665 M](https://img.qammunity.org/2021/formulas/chemistry/college/jyhd26otp7jffgchwzvodrg7omsgfzy564.png)
Now, we have the following dissociation of H₂SO₃ in water:
H₂SO₃(ac) + H₂O(l) ⇄ HSO₃⁻(ac) + H₃O⁺(ac) Ka₁ = 1.5x10⁻²
0.665-x x x
![Ka_(1) = ([HSO_(3)^(-)][H_(3)O^(+)])/([H_(2)SO_(3)]) = 1.5\cdot 10^(-2)](https://img.qammunity.org/2021/formulas/chemistry/college/4uoipvv8yz5fkodsb262dgqs7gfdj2npz4.png)
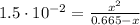
Solving the above equation for x we have:
x = 0.093 = [H₃O⁺] = [HSO₃⁻]
Also, the HSO₃⁻ dissociates in water as follows:
HSO₃⁻(c) + H₂O(l) ⇄ SO₃²⁻(ac) + H₃O⁺(ac) Ka₂ = 6.3x10⁻⁸
0.093-y y 0.093+y
![Ka_(2) = ([SO_(3)^(2-)][H_(3)O^(+)])/([HSO_(3)^(-)]) = 6.3\cdot 10^(-8)](https://img.qammunity.org/2021/formulas/chemistry/college/dowdae6t7cc9qiz09zcvbydyej71f7zunb.png)
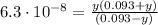
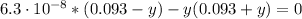
Solving the above equation for y we have:
y = 6.3x10⁻⁸
So, the concentration of H₃O⁺ is:
![[H_(3)O^(+)] = 0.093 M + 6.3\cdot 10^(-8) M = 0.093 M](https://img.qammunity.org/2021/formulas/chemistry/college/8tyxzlhiabzkapaixbxlqpenq702i6s5eq.png)
Finally, the pH is:
![pH = -log[H_(3)O{+}] = -log(0.093) = 1.03](https://img.qammunity.org/2021/formulas/chemistry/college/stylvo3ytetwyy8673y5285sd4zjn3hiti.png)
Therefore, the pH in a solution prepared by continuously bubbling SO₂ is 1.03.
I hope it helps you!