Answer:
43.2 mL
Step-by-step explanation:
The reaction is:
5H₂C₂O₄ + 2MnO₄⁻ + 6H⁺ → 10CO₂ + 2Mn²⁺ + 8H₂O
We have:
C(KMnO₄) = 0.1650 M
V(KMnO₄) =?
C(H₂C₂O₄) = 0.165 M
V(H₂C₂O₄) = 108.0 mL
From equation (1) we have that 5 moles of H₂C₂O₄ react with 2 moles of MnO₄⁻, so the number of moles of KMnO₄ is:
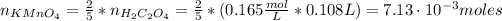
Now, we can find the volume of KMnO₄ as follows:

Therefore, are needed 43.2 mL of KMnO₄.
I hope it helps you!