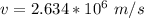Answer:
Step-by-step explanation:
From the question we are told that
The mass of chlorine ion is
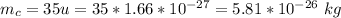
The charge is
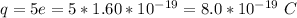
The potential difference is
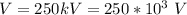
The radius of curvature of the path is
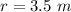
Gnerally the magnetic force will cause the speed of the chlorine ions to change from 0 m/s to
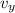 m/s along the y -axis but will not affect the velocity along the x-axis
m/s along the y -axis but will not affect the velocity along the x-axis
Generally according the law of energy conservation
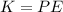
Here K is the kinetic energy of the of the chlorine ions which is mathematically represented as
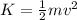
And PE is electric potential energy which is mathematically represented as
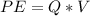
So
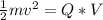
=>
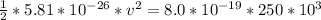
=>
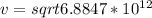
=>