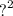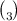In determining the percentage purity of an impure NaCo3 for 4.2g of the impure was place in measuring cylinder to work up to 1000cm . the resultant solution is neutralize by 0.092m of 20.40cm3 Hcl in lab [ na=23,o= 16, c=12, h=1, cl=35-5]
hint: percentage purity =mass pure substance ×100
mass concentrate : impure substance??