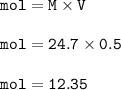Mol of methanol =12.35
Further explanation
Mole itself is the number of particles contained in a substance
1 mole = 6.02.10²³ particles
Mole : the ratio of the amount of substance mass and its molar mass
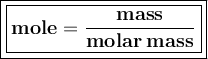
Molarity is a way to express the concentration of the solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
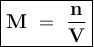
Molar concentration of methanol=24.7 M
Volume of solution = 500 ml = 0.5 L