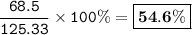%yield = 54.6%
Further explanation
Percent yield is the compare of the amount of product obtained from a reaction with the amount you calculated
(theoretical)
General formula:
Percent yield = (Actual yield / theoretical yield )x 100%
Reaction
2ZnS+3O₂ ⇒ 2ZnO+2SO₂
MW ZnS = 97.474 g/mol
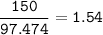
MW ZnO = 81.38 g/mol
- mol ZnO (from mol ZnS as limiting reactant, O₂ excess)
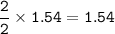
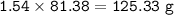
Theoretical production = 125.388