Answer:
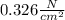
Step-by-step explanation:
Hello.
In this case, considering that 1 atm equals 1.01 kPa, we can compute the pressure in kPa first as shown below:
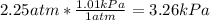
Now, we convert kPa to Pa, considering 1 kPa equals 1000 Pa:
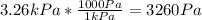
Now, since Pa is equal to N/m², and 1 m equals 100 cm, the pressure in newton per square centimeter turns out:
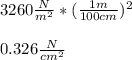
Best regards.